This web page was produced as an assignment for Genetics 677, an undergraduate course at UW-Madison.
Protein Network
MYC Protein Interaction
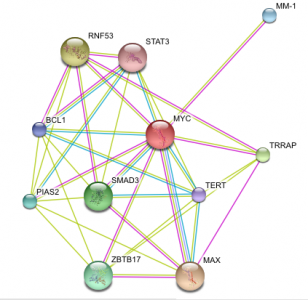
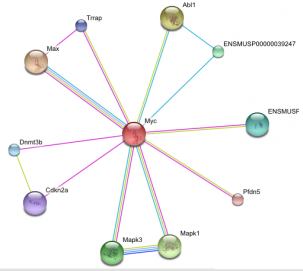
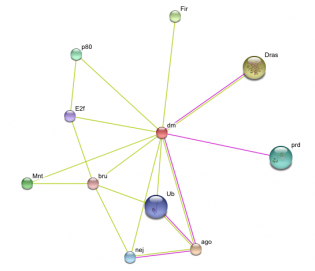
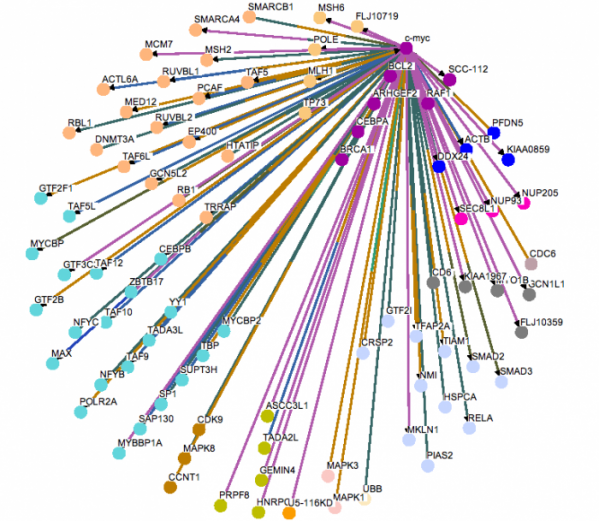
Above are images of MYC protein interaction networks obtained from String. Each colored line represents a different means of evidence that was obtained for each interaction.(1) For complete details of how the interaction was proven and what each of the protein abbreviations stand for please visit the following link. MYC String Interaction. The above most image is of the MYC interactions in humans. The image on the left (directly above text) shows the protein network of MYC found in mice. On the right (again, directly above text) is of the MYC homolog, diminutive (dm), found in the fruit fly. Probably one of the most important interactions (and the one with the most evidence supporting the interaction) is that of MYC and the MYC-associated factor X (MAX). This interaction is found in both humans and in mice. As stated on the Protein Data page, it is the MYC-MAX heterodimer that is capable of interacting with the genes responsible for cell cycle control, apoptosis, and a variety of other diverse functions. If one were to look at the protein domains in the MAX protein and compare them to the domains present on the Mnt protein found in fruit flies, it would be observed that both contain a helix-loop-helix domain which is present in all eukaryotic transcription factors which includes MYC and dm. For a little bit more information concerning Mnt, see the Phenotypes and Gene Ontology page. Another interaction that is of some importance is the MM-1 protein also known as prefoldin subunit 5 (PFDN5) as it is found in mouse protein network. MM-1 (PFDN5) is a chaperone protein that participates in the correct folding of polypeptides after they are synthesized. The interaction between MM-1 and MYC, through experiments and textmining, has been proposed to negatively influence (repress) the transcriptional activity of MYC.(5) Future work with MM-1 and MYC may prove useful in controlling unregulated proliferation of cells regulated under MYC.(2) The presence of Smad3 and Mnt in the human and fly networks, respectfully, should also have some light shed on them. Mnt is an ortholog of the Mad family in which Smad3 belongs and studying Mnt in flies may be of some use in a further understanding of the functioning of MYC in humans. Smad3 is capable of forming a complex with Sp1 (which MYC contains a domain of. See Gene Sequence). MYC is then capable of inihibiting the inhibition of growth that results from the Smad and Sp1 complex on TGF-beta, a trasnforming growth factor.(1)(6)
Protein Pathways
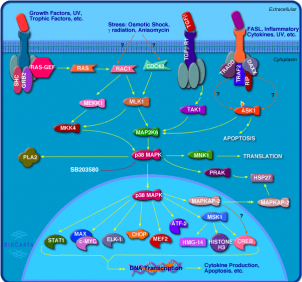
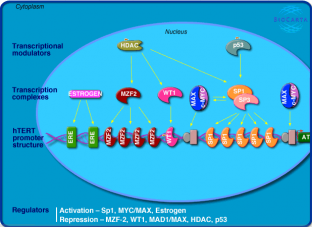
MYC Cellular Pathways
Above are images of two pathways that MYC and MAX interact with one another.(3) Click on the images for a more detailed description of each of the pathways.
For more pathways involving MYC follow the below links.(3)
Cadmium induces DNA synthesis and proliferation in macrophages
CTCF: First Multivalent Nuclear Factor
Erk1/Erk2 Mapk Signaling pathway
IL-2 Receptor Beta Chain in T cell Activation
Inhibition of Cellular Proliferation by Gleevec
MAPKinase Signaling Pathway
Neuropeptides VIP and PACAP inhibit the apoptosis of activated T cells
Role of EGF Receptor Transactivation by GPCRs in Cardiac Hypertrophy
Telomeres, Telomerase, Cellular Aging, and Immortality
Tumor Suppressor Arf Inhibits Ribosomal Biogenesis
WNT Signaling Pathway
All these pathways display the wide variety of functions that MYC has a role in.
References
1String
2Entrez Protein
3BioCarta
4Osprey
5Hagio, Y., Kimura, Y., and Taira, T. (2006) Distinct localizations and repression activities of MM-1 isoforms toward c-Myc. Journal of Biological Chemistry. 97(1):145-55.
6Feng, X., Liang, Y., and Liang, M.(2002).Direct Interaction of c-Myc with Smad2 and Smad3 to Inhibit TGF-β-Mediated Induction of the CDK Inhibitor p15Ink4B. Molecular Cell. 9(1):133-143. Retrieved from: http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WSR-4C5RFFB-J&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5ed8f5bd595b7103fdbe1b0aa055b850
Contact Info: Deeter Neumann, [email protected], May 14, 2009